-
×
 Buy Farmapram bars : Mexican pharmaceuticals
1 × £90.00
Buy Farmapram bars : Mexican pharmaceuticals
1 × £90.00 -
×
 Buy ADDERALL XL 30g (90) Extended Release Capsules
1 × £150.00
Buy ADDERALL XL 30g (90) Extended Release Capsules
1 × £150.00 -
×
 4g Colombian Fishscale Cocaine (96% Pure)
1 × £250.00
4g Colombian Fishscale Cocaine (96% Pure)
1 × £250.00
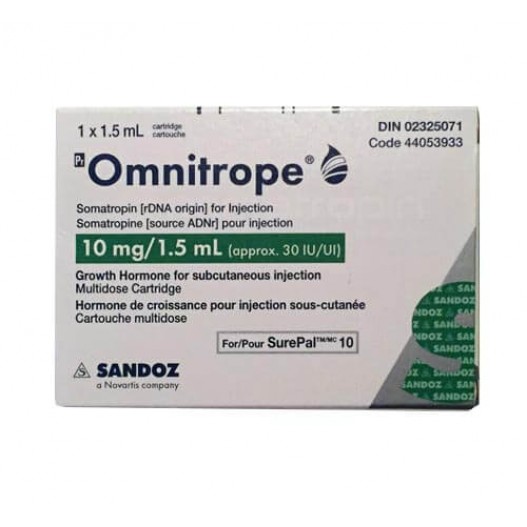
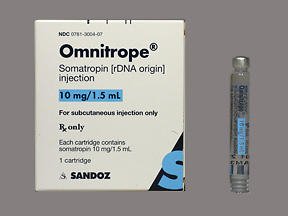
Buy Omnitrope Cartridge Online
£300.00 Original price was: £300.00.£270.00Current price is: £270.00.
Category: Human Growth Hormone
DESCRIPTION
Omnitrope is a synthetic form of human growth hormone (HGH). HGH is a naturally occurring protein in the body; it stimulates growth of the bone and cartilage. Omnitrope is a recombinant protein, i.e. it is produced under laboratory conditions and is structurally almost identical to the natural HGH. It is an FDA-approved easy-to-use product, and is a cost-effective treatment for children with growth hormone deficiency.
In paediatrics, Omnitrope is prescribed for children with growth failure due to growth hormone deficiency (GHD) and Prader-Willi syndrome (diagnosed by genetic testing). It is also indicated for the treatment of SGA growth failure in children born small for gestational age, failing to catch-up growth by the age of 2, as well as for children that do not grow due to idiopathic short stature (with no known cause) and ones suffering from Turner Syndrome (females with only a single X-chromosome). In adults, Omnitrope is used to treat adult onset or childhood onset growth hormone deficiency.
Omnitrope is contraindicated in children: with acute critical illnesses, with respiratory failure, with cancer/ active malignancy, with active proliferative or non-proliferative diabetic retinopathy, with known hypersensitivity to Somatropin, with closed epiphyseal plates, who are severely obese or with respiratory impairment with Prader-Willi Syndrome, after trauma, post-surgery.
Before prescribing Omnitrope, doctor should look out for the following: Signs of upper airway obstruction and sleep apnoea in children with Prader-Willi Syndrome; if any symptoms arise treatment should be discontinued; Neoplasm: patients with pre-existing tumours should be monitored for progression or recurrence; childhood cancer survivors if treated with somatropin, hold the increased risk of a second neoplasm, especially meningiomas in those treated with radiation to the head for their first neoplasm; Glucose levels should be monitored in all patients; in diabetic patients, dosage of concurrent anti-hyperglycaemic may require adjustment as impaired glucose tolerance and diabetes mellitus may get unmasked; Intracranial Hypertension may develop; can be reversed by reducing the dose or terminating the treatment; Fluid retention – frequent in adults, like oedema, arthralgia or carpal tunnel syndrome. Dose reduction is required in such cases; Other hormone replacement therapies should be monitored closely due to an increased risk for hypopituitarism; Hypothyroidism can become evident or get worse; Children with the onset of a limp or hip/knee pain should be evaluated for slipped capital femoral epiphysis Pre-existing scoliosis may worsen Pancreatitis should be considered in patients with persistent severe abdominal pain.
Formulations containing BA (benzyl alcohol) should not be used in premature babies or neonates (e.g., 5 mg/1.5 mL Omnitrope + Bacteriostatic Water diluent for the Omnitrope. Once the treatment is started, risks of continuing Omnitrope should be considered if the child has any of the following: scoliosis, diabetes, cancer, hormone deficiencies, trauma, complains about hip or knee pains, limping, or difficulty breathing. Tests for child’s blood glucose and funduscopic examination (an eye test for intracranial hypertension) must be performed before starting the treatment and periodically afterwards. Parents of children, who are treated for Prader-Willi syndrome and start having difficulty breathing, start snoring or their snoring increases, should seek medical advice.
Side effects that may arise after the treatment with Omnitrope include headaches, loss of fatty tissue under the skin (lipoatrophy) or rash at the injection sites.
Be the first to review “Buy Omnitrope Cartridge Online” Cancel reply
Related products
Sale!
Human Growth Hormone
Human Growth Hormone
£80.00
Sale!
Human Growth Hormone
Human Growth Hormone
£250.00
Sale!
Human Growth Hormone
Sale!
Human Growth Hormone
Sale!
Human Growth Hormone
Sale!
Human Growth Hormone






Reviews
There are no reviews yet.